General isotherm info#
Before we start the characterisation, let's have a cursory look at the isotherms. First, make sure the data is imported by running the previous notebook.
[1]:
# import isotherms
%run import.ipynb
Selected 5 isotherms with nitrogen at 77K
Selected 2 room temperature calorimetry isotherms
Selected 2 isotherms for IAST calculation
Selected 3 isotherms for isosteric enthalpy calculation
We know that some of the isotherms are measured with nitrogen at 77 kelvin, but don't know what the samples are. If we evaluate an isotherm, its key details are automatically displayed.
[2]:
isotherms_n2_77k
[ <PointIsotherm 5b36aabf643c8557893f1a82aeedffaa>: 'nitrogen' on 'MCM-41' at 77.355 K, <PointIsotherm e0590c7a3dae0ea88d7328c2c1a31fed>: 'nitrogen' on 'NaY' at 77.355 K, <PointIsotherm 25abc19b921164e130f13e0126763853>: 'nitrogen' on 'SiO2' at 77.355 K, <PointIsotherm b72b8b4b525dc901d2977ad577637462>: 'nitrogen' on 'Takeda 5A' at 77.355 K, <PointIsotherm 5133355996d3f32458515fd1cf492813>: 'nitrogen' on 'UiO-66(Zr)' at 77.355 K ]
So we have a mesoporous templated silica, a zeolite, some amorphous silica, a microporous carbon and a common MOF.
What about the isotherms which we'll use for isosteric calculations? Let's see what is the sample and what temperatures they are recorded at. We can use the standard print method on an isotherm for some detailed info.
[3]:
print(isotherms_isosteric[0])
print([isotherm.temperature for isotherm in isotherms_isosteric])
Material: TEST
Adsorbate: n-butane
Temperature: 298.15K
Units:
Uptake in: mmol/g
Pressure in: bar
Other properties:
iso_type: isotherm
material_batch: TB
[298.15, 323.15, 348.15]
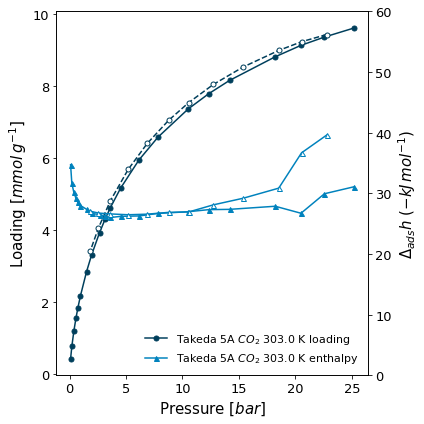
Let's look at the isotherms that were measured in a combination with microcalorimetry. Besides the loading and pressure points, these isotherms also have a differential enthalpy of adsorption measured for each point. We can use the isotherm.print_info function, which also outputs a graph of the isotherm besides its properties.
[4]:
# The second axis range limits are given to the print function
isotherms_calorimetry[1].print_info(y2_range=(0,60))
Material: Takeda 5A
Adsorbate: carbon dioxide
Temperature: 303.0K
Units:
Uptake in: mmol/g
Pressure in: bar
Other properties:
iso_type: Calorimetrie
lab: MADIREL
instrument: CV
material_batch: Test
activation_temperature: 150.0
user: ADW
( <AxesSubplot:xlabel='Pressure [$bar$]', ylabel='Loading [$mmol\\/g^{-1}$]'>, <AxesSubplot:ylabel='$\\Delta_{ads}h$ $(-kJ\\/mol^{-1})$'> )
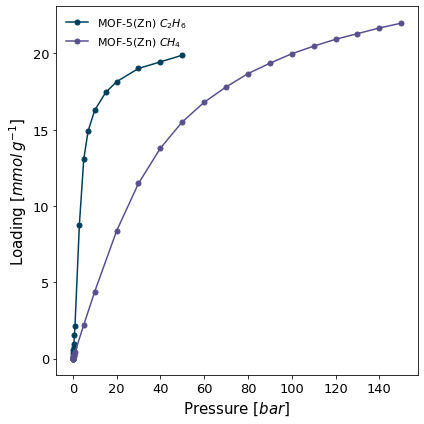
For the isotherms which are to be used for IAST calculations, we'd like to plot them on the same graph, with the name of the adsorbate in the legend. For this we can use the more general pygaps.graphing.plot_iso function.
[5]:
# import the characterisation module
import pygaps.graphing as pgg
pgg.plot_iso(
isotherms_iast, # the isotherms
branch='ads', # only the adsorption branch
lgd_keys=['material','adsorbate'], # the isotherm properties making up the legend
)
<AxesSubplot:xlabel='Pressure [$bar$]', ylabel='Loading [$mmol\\/g^{-1}$]'>