Sorption enthalpy calculations#
Sorption enthalpy, \(\Delta H_{ads}\), is an indication of the strength of the adsorbate-material interaction and can be estimated through several methods.
Isosteric enthalpy (Clausius-Clapeyron)#
In order to calculate \(\Delta H_{ads}\), at least two isotherms which were taken at different temperatures are required.
First, make sure the data is imported.
[1]:
# import isotherms
%run import.ipynb
%matplotlib inline
# import the characterisation module
import pygaps.characterisation as pgc
Selected 5 isotherms with nitrogen at 77K
Selected 2 room temperature calorimetry isotherms
Selected 2 isotherms for IAST calculation
Selected 3 isotherms for isosteric enthalpy calculation
The file version is None while the parser uses version 1.0. Strange things might happen, so double check your data.
Could not parse parameter material_mass, currently unknown
Let's quickly plot the isotherms to see how they look. We put the temperature of the experiment in the legend by using the lgd_keys keyword.
[2]:
# import the graphing module
import pygaps.graphing as pgg
pgg.plot_iso(
isotherms_isosteric,
lgd_keys=['adsorbate', 'temperature'],
)
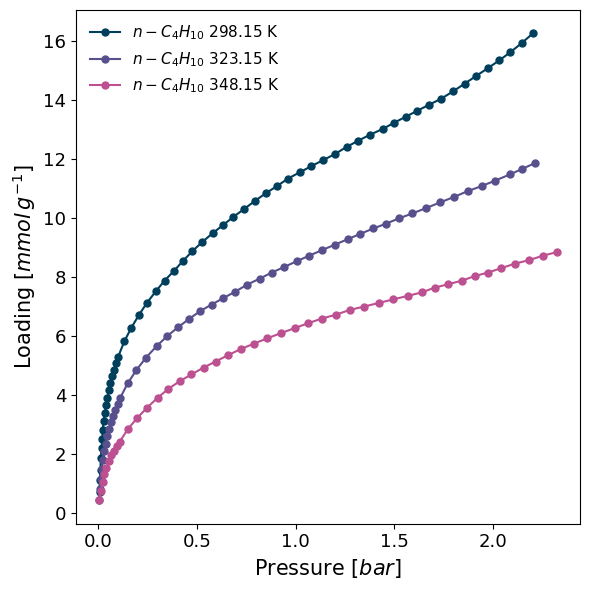
The isotherms look good, except perhaps a bit of measurement error in the low pressure region.
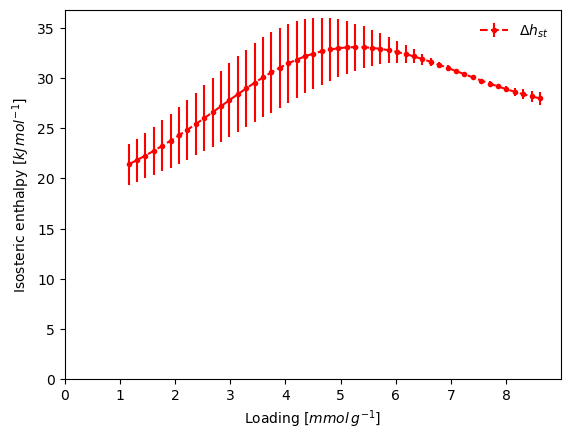
The isosteric enthalpy calculation takes the list of the isotherms and returns the results as a dictionary. Using the verbose keyword, we also generate a graph that includes an error bar.
[3]:
result_dict = pgc.isosteric_enthalpy(isotherms_isosteric, verbose=True)
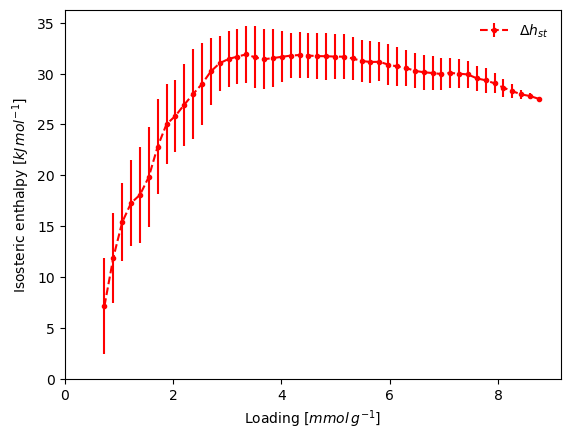
The inaccuracy in the low pressure region has contributed to the odd enthalpy curve. One other option would be to first fit a model to each isotherm, then use it for the enthalpy determination.
Let's try a Double Site Langmuir model and then re-run the isosteric calculation.
[4]:
from pygaps.modelling import model_iso
models_isosteric = [
model_iso(iso, model="dslangmuir")
for iso in isotherms_isosteric
]
result_dict = pgc.isosteric_enthalpy(models_isosteric, verbose=True)
More information about the functions and their use can be found in the manual.
Whittaker method#
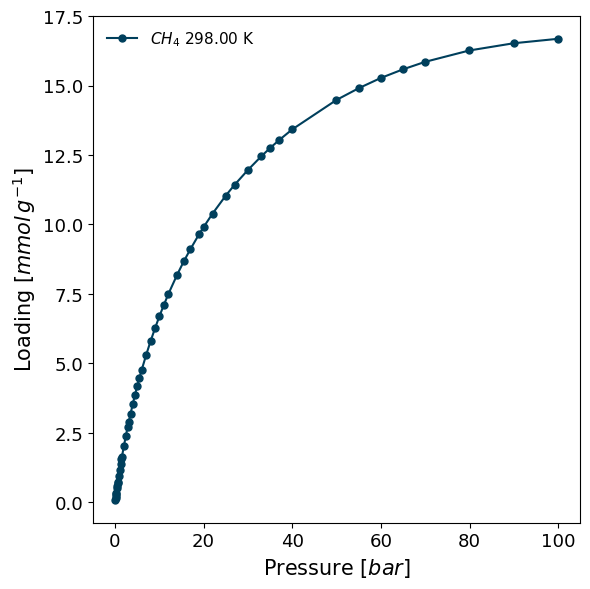
The Whittaker method uses a Tóth’s modification of Polanyi's potential theory to determine enthalpy of adsorption from a single isotherm fitted with a suitable model. We loaded the example isotherm before, so we can now plot it.
[5]:
pgg.plot_iso(
isotherms_enth_whittaker,
lgd_keys=['adsorbate', 'temperature'],
pressure_unit='bar'
)
The code can accept a ModelIsotherm of the required type. Note that this isotherm must be in correct pressure mode (absolute) and units: (Pa).
[6]:
from pygaps.modelling import model_iso
for iso in isotherms_enth_whittaker:
iso.convert_pressure(mode_to="absolute", unit_to="Pa")
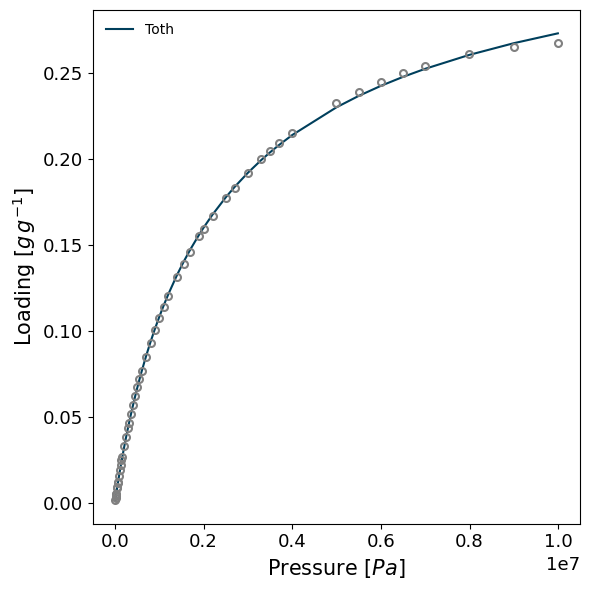
models_whittaker = model_iso(isotherms_enth_whittaker[0], model="Toth")
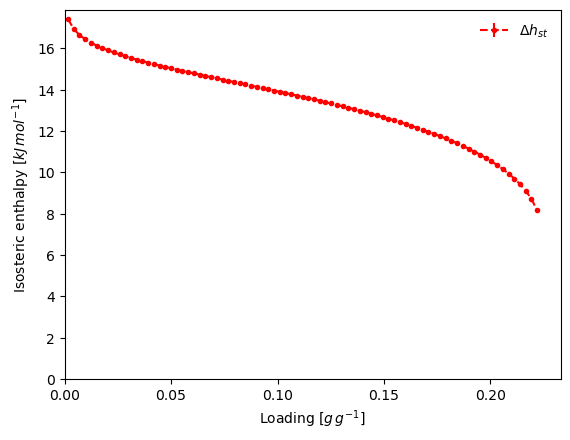
result_dict = pgc.enthalpy_sorption_whittaker(models_whittaker, verbose=True)
Thermodynamic backend failed with error: Temperature to QT_flash [298 K] must be in range [90.5941 K, 190.564 K]. Attempting to read parameters dictionary...
methane does not have a saturation pressure at 298.0 K. Calculating pseudo-saturation pressure...
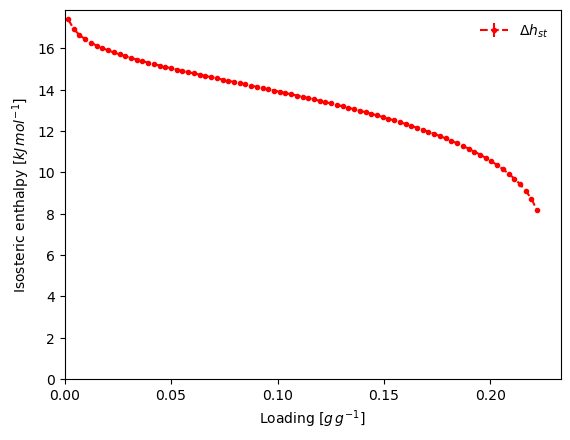
Alternatively, the PointIsotherm and the desired model can be passed as parameters, and fitting is performed automatically before the method is applied.
[7]:
result_dict = pgc.enthalpy_sorption_whittaker(isotherms_enth_whittaker[0], model="Toth", verbose=True)
Attempting to model using Toth.
Model Toth success, RMSE is 0.00493
Thermodynamic backend failed with error: Temperature to QT_flash [298 K] must be in range [90.5941 K, 190.564 K]. Attempting to read parameters dictionary...
methane does not have a saturation pressure at 298.0 K. Calculating pseudo-saturation pressure...